Background:
CAR T-cell therapy has transformed the treatment of hematologic malignancies, but challenges persist due to toxicities like cytokine release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS), and infections. Rates of invasive fungal infections (IFI) post CAR T-cell therapy are estimated to range from 1% to 7% (Haidar et al. Clin Infect Dis, 2020). Some centers use positive fungal biomarkers, such as serum (1à3)-β-D-glucan (B-DG) and galactomannan (GM), alongside surveillance radiographic imaging to guide preemptive fungal therapy in this population. The objective of the study was to assess the diagnostic performance of surveillance B-DG and GM for the diagnosis of IFI in recipients of BCMA and CD19-directed CAR T for multiple myeloma (MM) and non-Hodgkin lymphoma (NHL), respectively.
Methods:
We conducted a retrospective analysis on adult patients who received BCMA-directed CAR T therapy for MM or CD19-directed CAR T for NHL at our institution between 2018-2023. IFI was defined using the Revised European Organization for Research and Treatment of Cancer and the Mycoses Study Group (EORTC/MSG) criteria (Donnelly et al. Clin Infect Dis, 2020). Only patients with proven or probable infection were included in the analysis. The choice of antifungal prophylaxis was determined based on the treating physician's discretion. In the most cases, B-DG and GM monitoring was done prior to apheresis, before CAR T infusion, and subsequently every 14 days for a duration of 6 months.
All patients were followed until 7/12/2023, or until their data were censored at the last follow-up. The cumulative incidence was calculated using Kaplan-Meier method, considering the time to IFI, with IFI-free death and disease relapse as competing risks. Statistical analysis was performed using IBM SPSS (version 29.0).
Result:
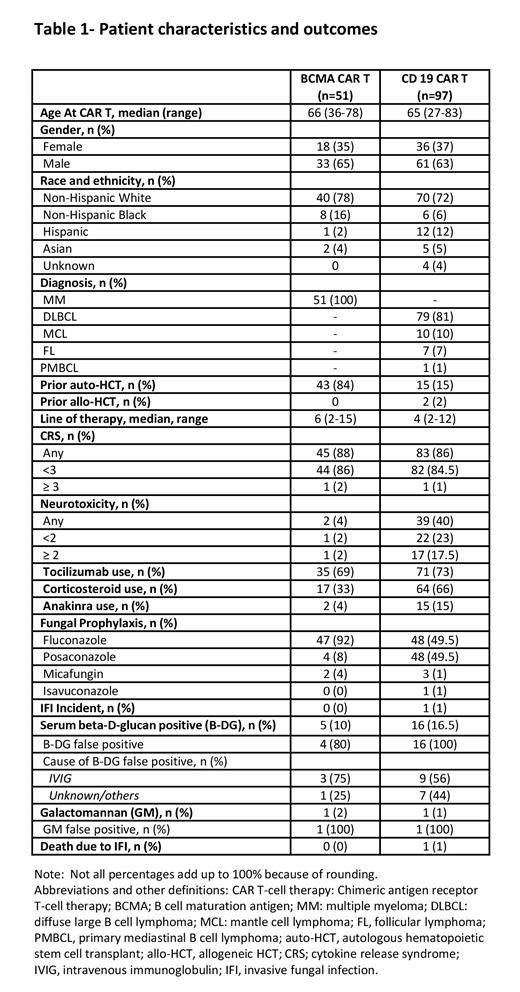
Among the 148 subjects in this study, 51 patients (34.5%) received BCMA CAR T, and 97 (65.5%) received CD19 CAR T therapy. The median age at CAR T infusion was 66 years (range 36-78) in the BCMA cohort and 65 years (range 27-83) in the CD19 cohort (p=0.64). The BCMA cohort had a higher number of prior therapies, with a median of 6 (range 2-15) compared to the CD19 cohort, which had a median of 4 (range 2-12) (p<0.001). The median follow-up from CAR T infusion was 9.5 months (range 0-63) in both cohorts, with 12 months (range 0-63) in the BCMA cohort and 8 months (range 0-49) in the CD19 cohort.
All patients received prophylactic antifungal agents, including anti-yeast (fluconazole, n=95), and anti-mold (micafungin n=5, isavuconazole n=1, or Posaconazole n=52). Among the BCMA cohort, 92% of the patients received anti-yeast prophylaxis, while 8% were given anti-mold prophylaxis. In the CD19 cohort, 49.5% received anti-yeast prophylaxis, while 50.5% received anti-mold prophylaxis. Out of the 148 patients, 21 individuals (14%) exhibited elevated B-DG levels, while only 2 patients (1%) showed elevated GM levels.
However, 20 out of 21 (95%) cases with positive B-DG, and all cases with positive GM were deemed to be false positives. Among these false positives, 60% were attributed to IVIG administration, while the cause for the remaining cases remained unknown (Table 1).
Only one patient in this study developed IFI. The patient received anti-yeast prophylaxis (Fluconazole) at CAR-T and had a disseminated Rhizopus infection on day 13 post CD19 CAR T, which ultimately resulted in the patient's death. The 1-year cumulative incidence of IFI, considering competing risks, was 0.7% (95% confidence interval [CI], 0- 2.1).
The median progression-free survival (PFS) in the overall cohort was 11.7 months (95% CI, 6.9-16.6), with 8.6 months (95% CI, 3.2-13.97) in the BCMA cohort and 14.6 months (95% CI, NR-NR) in the CD19 cohort (p=0.324). The median overall survival (OS) of the study cohort was 32.4 months (95% CI, 16.3-48.4), with 41.4 months (95% CI, 16.74-66.08) in the BCMA cohort and 23.98 months (95% CI, NR-NR) in the CD19 cohort (p=0.260).
Conclusion:
The rate of IFI in our cohort was low (0.7%) post-CAR T therapy. The diagnostic performance of serial monitoring of B-DG and GM for the diagnosis of IFI is poor in recipients of BCMA-directed CAR T therapy for MM or CD19-directed CAR T for NHL.
Disclosures
Madanat:MD Education: Honoraria; OncLive: Honoraria; Novartis: Honoraria; Taiho oncology: Honoraria; Stemline therapeutics: Honoraria; Morphosys: Honoraria, Other: travel reimbursement; Sierra Oncology: Honoraria; GERON: Consultancy; Blueprint Medicines: Consultancy, Honoraria, Other: travel reimbursement; Rigel Pharmaceuticals: Honoraria. Kaur:Cellectar: Consultancy; Abbvie: Research Funding; Arcellx: Consultancy, Research Funding; Sanofi: Consultancy; Janssen: Consultancy, Research Funding; Pfizer: Consultancy; Kedrion: Consultancy; BMS: Consultancy, Research Funding. Anderson:Beigene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GlaxoSmithKline: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Cellectar: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Prothena: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Awan:Pharmacyclics LLC, an AbbVie Company.: Other: Contracted Research; Janssen, Gilead, Kite pharmaceuticals, Karyopharm, MEI Pharma, Verastem, Incyte, Johnson and Johnson, Merck, Epizyme, Loxo Oncology, Adaptive Biotechnologies, Genmab: Other: Consulting Agreements; AstraZeneca Pharmaceuticals LP: Other: Advisory Committee; AbbVie Inc, ADC Therapeutics, AstraZeneca Pharmaceuticals LP, BeiGene Ltd, Bristol-Myers Squibb Company, Cardinal Health, Caribou Biosciences Inc, Celgene Corporation, Cellectar Biosciences Inc, DAVA Oncology, Epizyme Inc, Genentech, a member of the Roche: Other: Consulting Agreements. La Hoz:Takeda: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal